Biomass energy production is pivotal in the sustainable energy landscape, leveraging organic materials to generate electricity, heat, and biofuels. This article delves into the essential components of biomass energy, including biofuel characterization and the primary conversion technologies: thermochemical and biochemical.
Source: www.iwatec.co.jp
1. Biofuel Characterization
Effective biomass energy production requires an understanding of biofuel properties.

Key characteristics include:
- Moisture Content – Represents the total water percentage in biomass. High moisture content reduces energy efficiency, as more energy is required to evaporate the water.
- Organic Matter and Ash Content – Organic matter contributes to energy production, while ash represents inorganic residue left after combustion. Lower ash content generally indicates higher fuel efficiency.
- Heating Value – Biofuels’ energy potential is measured as High Heating Value (HHV) and Low Heating Value (LHV), which reflect energy release during combustion:
- HHV assumes that water in the fuel is condensed after combustion, reclaiming the latent heat of vaporization.
- LHV assumes water remains vaporized, omitting latent heat.
- Elemental Composition – Carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and sulfur (S) are primary elements in biofuels. Biomass fuels have higher oxygen and volatile contents than coal, which impacts their combustion behaviour.
- For example:
- Carbon (C): 30-50%
- Hydrogen (H): 3-6%
- Oxygen (O): 20-50%
- Sulfur (S): less than 1%
- For example:
2. Thermochemical Conversion
Thermochemical conversion uses heat to convert biomass into energy, which can occur in the presence or absence of oxygen or other gasifying agents. The primary methods are combustion, gasification, and pyrolysis.
a. Combustion
Combustion is a highly exothermic reaction where biomass reacts with oxygen to release heat, which can be used directly or to produce steam for electricity. The general combustion reaction is:

In this equation:
- CxHyOz represents a generic biomass fuel with specific carbon, hydrogen, and oxygen ratios.
- The products include carbon dioxide (CO₂), water vapor (H₂O), and nitrogen (N₂).
Key factors affecting combustion include:
- Air-to-Fuel Ratio (AFR): AFR dictates whether there is sufficient oxygen for complete combustion.
- Stoichiometric AFR achieves complete combustion, where all carbon converts to CO₂ and hydrogen to H₂O.
- Excess Air (AFR > stoichiometric) ensures complete combustion but can reduce efficiency by cooling the flame.
- Equivalency Ratio (λ): Defined as the ratio of actual AFR to the stoichiometric AFR, it indicates combustion conditions:
- λ=1 for stoichiometric conditions
- λ>1 for fuel-lean (excess air)
- λ<1 for fuel-rich (insufficient air)

Source: https://www.youtube.com/watch?v=pd9zCb-exkU
b. Gasification
Gasification is a partial oxidation process that converts biomass into a synthesis gas (syngas) containing hydrogen (H₂), carbon monoxide (CO), methane (CH₄), and other compounds. This process occurs with limited oxygen, making it endothermic. The syngas can be used as a fuel for engines or further refined into chemicals or fuels.
ypical gasification reactions include:
- Partial Oxidation: Produces mainly CO and H₂

- Water-Gas Reaction: Enhances H₂ production through the reaction of carbon with water vapor:

Gasification allows flexibility, as the resulting syngas can be purified and transformed into valuable products via Fischer-Tropsch synthesis or other processes.
Source: https://www.youtube.com/watch?v=bPRa31dS0vA
c. Pyrolysis
Pyrolysis is the thermal decomposition of biomass in the absence of oxygen, producing bio-oil, char, and syngas. The process is versatile, with different types fast, intermediate, and slow pyrolysis each producing varying yields of bio-oil, char, and gas:
- Fast Pyrolysis: Heats biomass rapidly to 400-500°C, producing high yields of bio-oil, a potential crude oil substitute.
- Slow Pyrolysis: Uses lower heating rates and longer residence times, maximizing char production, useful for soil enhancement or carbon sequestration.
The typical products of pyrolysis include:
- Liquid (Bio-Oil): Approximately 20% water content, containing various organic compounds with a heating value of 13-18 MJ/kg.
- Gas: Mixture of CO₂, CO, CH₄, and other light hydrocarbons.
- Char: High carbon content with a heating value of around 30 MJ/kg.
Source: https://www.youtube.com/watch?v=3K1zWAYDvMA
3. Biochemical Conversion
Biochemical conversion relies on microorganisms to break down biomass, turning it into biofuels. Key processes include anaerobic digestion and fermentation.
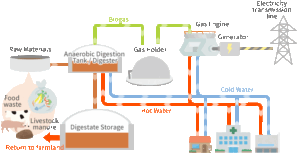
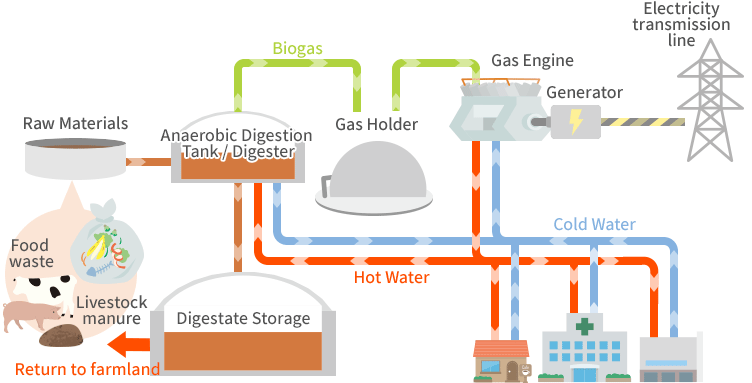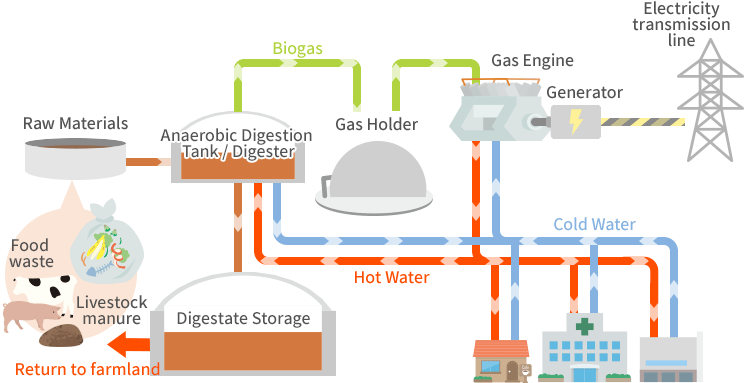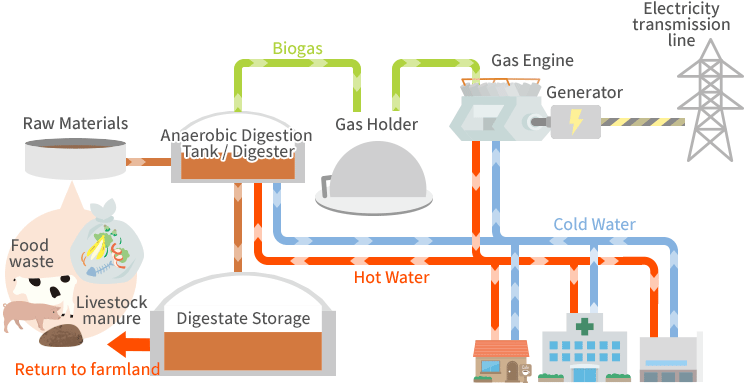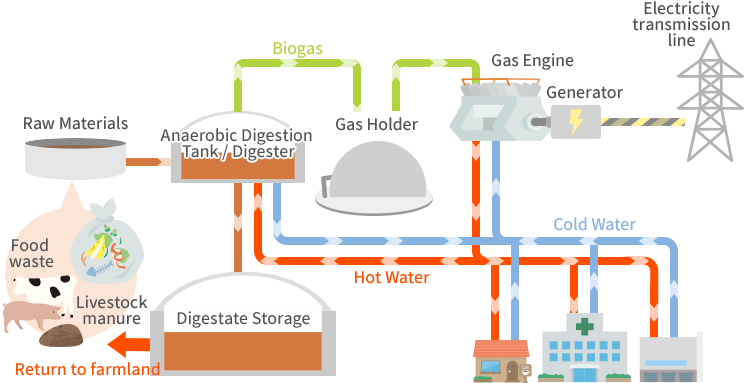
a. Anaerobic Digestion
Anaerobic digestion uses microbes to decompose organic material in the absence of oxygen, producing biogas, primarily methane (CH₄) and carbon dioxide (CO₂). The process involves several stages:
- Hydrolysis: Complex organic molecules (e.g., cellulose, proteins) are broken down into simpler molecules like sugars and amino acids.
- Acidogenesis and Acetogenesis: Intermediate compounds are further broken down by bacteria, producing volatile fatty acids, hydrogen, and acetic acid.
- Methanogenesis: Methanogenic bacteria convert these products into methane and carbon dioxide:

Anaerobic digestion’s efficiency depends on pH, temperature (mesophilic at 35-40°C or thermophilic at 55-60°C), and the carbon-to-nitrogen (C/N) ratio. The process is widely used for waste treatment and renewable methane production.
Source :https://www.youtube.com/watch?v=aULRryCVMyY
b. Fermentation
Fermentation uses microorganisms, typically yeast, to convert sugars into ethanol, a biofuel. The process involves several steps:
- Hydrolysis of Starches and Cellulose: Enzymes break down complex carbohydrates into simple fermentable sugars.
- Yeast Fermentation: Sugars undergo anaerobic fermentation, converting into ethanol and CO₂:

- Ethanol Recovery: The mixture is distilled to separate and purify ethanol for use as a fuel.
Ethanol fermentation can use first-generation (1G) feedstocks like corn or sugarcane, or second-generation (2G) lignocellulosic biomass, which includes agricultural residues and non-food crops. Second-generation bioethanol holds promise for sustainable fuel production by utilizing non-edible biomass sources.
Reference :
- Aldo Vieira da Rosa & Juan Carlos Ordóñez, Chapter 13 –Biomass, in Fundamentals of Renewable Energy Processes, 4th edition, Academic Press (2022).
- US Environmental Protection Agency, How Does Anaerobic Digestion Work? | US EPA
- MdMoslehUddin & Mark MbaWright, Anaerobic Digestion Fundamentals, Challenges, and Technical Advances, Physical Sciences Reviews, De Gruyter(2021).
Thank you for reading! We hope this article has enriched your understanding and inspired you to explore the subject further.